Short Biography
Liliane Michalik received her PhD from the University Louis Pasteur of Strasbourg in 1993, for work on microtubule-associated proteins in the group of Jean-François Launay, INSERM. In 1994, she joined the group of Hansjorg Keller, then Walter Wahli at UNIL for her post-doctoral training, during which she initiated a research project aimed at elucidating the roles of the nuclear hormone receptors PPARs in skin homeostasis and repair. Between 1996 and 2002, she persued her research in the same field as Maître Assistant, then Maître d’Enseignement et de Recherche at UNIL. She arrived at the Center for Integrative Genomics in 2003 as Maître d’Enseignement et de Recherche, is MER-privat docent since 2008 and Vice-director of the « Ecole de Biologie » since November 1st, 2011.

LILIANE MICHALIK – RESEARCH REPORT
PPAR-dependent control of the skin responses to environmental cues
Our genome constantly adapts its transcription to translate cues (e.g. nutrients, hormones) and insults (e.g. toxins, allergens, mechanical injuries, excessive sunlight (UV)) into cellular responses. Our research aims at understanding how our genome translates environmental insults into cellular responses. In that respect, PPARs are transcription factors of the nuclear hormone receptor family that act as sensors and tune cellular responses by regulating the transcription of their target genes. As the skin is our largest interface with the environment and our most critical protection, it is the ideal model organ to pursue our goal.
In mammals, there are four PPAR proteins (PPARα, PPARβ/δ -henceforth referred to as PPARβ-, and PPARγ1 and PPARγ2, referred together as PPARγ) encoded by three different genes. As nuclear hormone receptors, PPARs are activated by natural endogenous agonists (fatty acids and derivatives), but can also be regulated by synthetic agonists or antagonists, what makes them very attractive drugable targets. PPARα and PPARγ are the targets of marketed compounds, the fibrates (PPARα activators) and the glitazones (PPARγ activators), which are used to treat dyslipidemia and type 2 diabetes, respectively.
In the past years, we have explored how the PPAR nuclear hormone receptors control the skin’s responses to mechanical injuries, allergens and UV rays. We showed that in response to a skin injury, PPARα is crucial to maintain a normal inflammatory response, while the activation of its target by PPARβ is key to skin cell survival, proliferation and migration (Michalik et al., J Cell Biol 2001; Tan et al., Genes Dev 2001; DiPoi et al., MolCell 2002; Michalik et al., Mol Endo 2005; Tan et al., MCB 2007; Chong et al., J Cell Biol 2009). We then broaden our studies to the regulatory roles of PPARβ in response to allergens, with a focus on blood vessel remodeling, a hallmark of inflammatory disorders and allergies. Using two original mouse models specifically lacking PPARβ in the two major blood vessel cell types, we demonstrated that endothelial but not smooth muscle PPARβ is essential to blood vessel remodeling. Of note, the absence of endothelial PPARβ reduces skin allergic reaction, as well as systemic anaphylaxis, the most severe form of allergic reaction (Wawrzyniak et al., JACI 2015).
We then decided to compare and contrast the roles of PPARβ and PPARγ in skin cancers, the ultimate response of the skin to Ultra Violet (UV) rays. Sunlight UV rays are potent DNA damaging agents. Excessive exposure of the skin to UV ultimately results in skin cell malignant transformation, promoting squamous cell carcinoma, the most aggressive form of human skin cancer of keratinocyte origin, or melanoma, skin cancer of melanocytes, the pigmented cells of the skin. Thanks to an original mouse model, our past work has demonstrated that in response to UV, PPARβ activates transcription of target genes that promote squamous cell carcinoma progression in mouse skin (Montagner et al, EMBO Mol Med 2014). We identified the microRNA miRNA-21-3p as an indirect PPAR target, and demonstrated that miR21-3p promotes PPAR-induced skin inflammation that sustains cancer progression. We collected data suggesting that miR-21-3p is a promising therapeutic target for human skin inflammatory diseases and skin epithelial cancers (Degueurce et al, EMBO Mol Med 2016). While PPARβ is involved in the development of skin cancers of keratinocyte origin, we currently develop a line of study suggesting that PPARγ plays a role in human melanoma. In the coming years we will to focus on the skin’s responses to UV rays and how PPARs affect skin cell UV-induced malignant transformation by regulating transcription of their target genes.
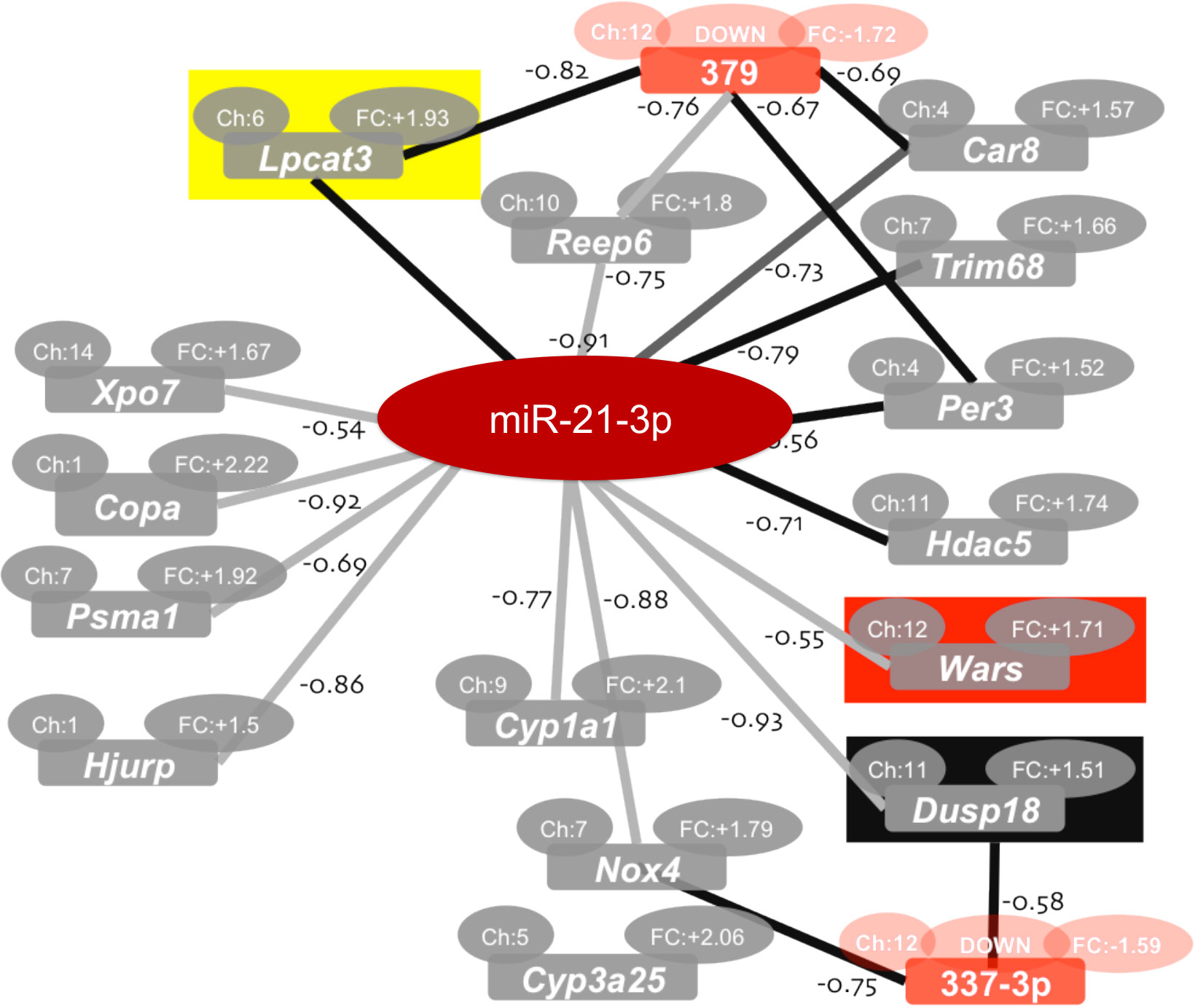
Fig 1. We have established that the passenger miRNA miR-21-3p is a novel UV-induced miRNA associated with skin cancer and skin inflammtory diseases. The inhibition of miR-21-3p reduces UV-induced cutaneous inflammation in ex vivo human skin biopsies, underlining the clinical relevance of miRNA-based topical therapies for cutaneous disorders.
GROUP MEMBERS
Group Leader
Liliane Michalik
liliane.michalik@unil.ch
Postdoctoral Fellows
Gwendoline Degueurce
Patrick Meylan
Christine Pich
PhD Student
Stefanie Ginster
Master Students
Alexandre Casanova
Fiona Marison
Undergraduate Student
Hélène Garcia
Technicians
Lisa Cherbuin
Lionel Mury
Marie Sgandurra
Administrative assistant
Carine Dovat
carine.dovat@unil.ch
