Short Biography
Richard Benton received his PhD in 2003 from the University of Cambridge, and was an EMBO/Heley Hay Whitney post-doctoral fellow at The Rockefeller University, New York. He joined the Center for Integrative Genomics in September 2007 as Assistant Professor and promoted to Associate Professor in August 2012. His group’s research has been recognised by award of the Eppendorf & Science Prize for Neurobiology (2009), Friedrich Miescher Award (2012), AChemS Young Investigator Award for Research in Olfaction (2012), the National Latsis Prize (2015), and the EMBO Gold Medal (2016). His research has been supported by the Swiss National Science Foundation, ERC Starting and Consolidator Grants, the EMBO Young Investigator Programme and an HFSP Young Investigator Award.

RICHARD BENTON – RESEARCH REPORT
Chemosensory perception and evolution in insects
We are interested in the genetic, neural and evolutionary basis of sensory perception. As a model, we investigate the chemosensory systems of the fruit fly Drosophila melanogaster, which control many sophisticated behaviours, but are experimentally highly accessible. Below we highlight some of our recent discoveries.
Evolutionary origin of insect chemosensory receptors
We have used comparative genomics to explore the origin of the insect chemosensory receptor superfamily, comprising the Odorant Receptors (ORs) and Gustatory Receptors (GRs). Although originally thought to be insect-specific genes, we identified distant relatives -“GR-like” (GRL) genes – in diverse animals, including non-Bilateria, as well as in plants. These findings indicate a very early origin of this gene family. Interestingly, expression and functional studies of GRLs in the sea anemone and sea urchin suggest that GRLs have developmental functions, arguing that the ancestral role of these proteins may not have been in chemosensation.
Chemosensory receptor population genetics
We have investigated the evolutionary forces affecting patterns of genetic variation over short-time scales within chemosensory gene repertoires – including OR, GRs and the Ionotropic Receptors (IRs) – using genome-wide data from geographically diverse populations. By couching population genomic analyses of these families within parallel analyses of other large gene families, we have demonstrated that chemosensory proteins are not outliers for adaptive divergence (between closely-related drosophilids), contrary to widespread belief that they are unusually rapidly evolving. However, chemosensory families often display the strongest genome-wide signals of recent selection within D. melanogaster, as genetic “first responders” to new ecological niches.
Olfactory receptor “pseudo-pseudogenes”
In the course of our analysis of the molecular evolution of IRs across drosophilids, we made an unexpected discovery: the functionality of a presumed pseudogenised receptor locus. This gene, Drosophila sechellia IR75a, bears a premature termination codon (PTC) that appears to be fixed in the population, but encodes a functional receptor due to efficient translational readthrough of the PTC. We identified several other examples of functional PTC- containing genes, suggesting that such “pseudo-pseudogenes” represent a widespread phenomenon that might include non-olfactory loci.
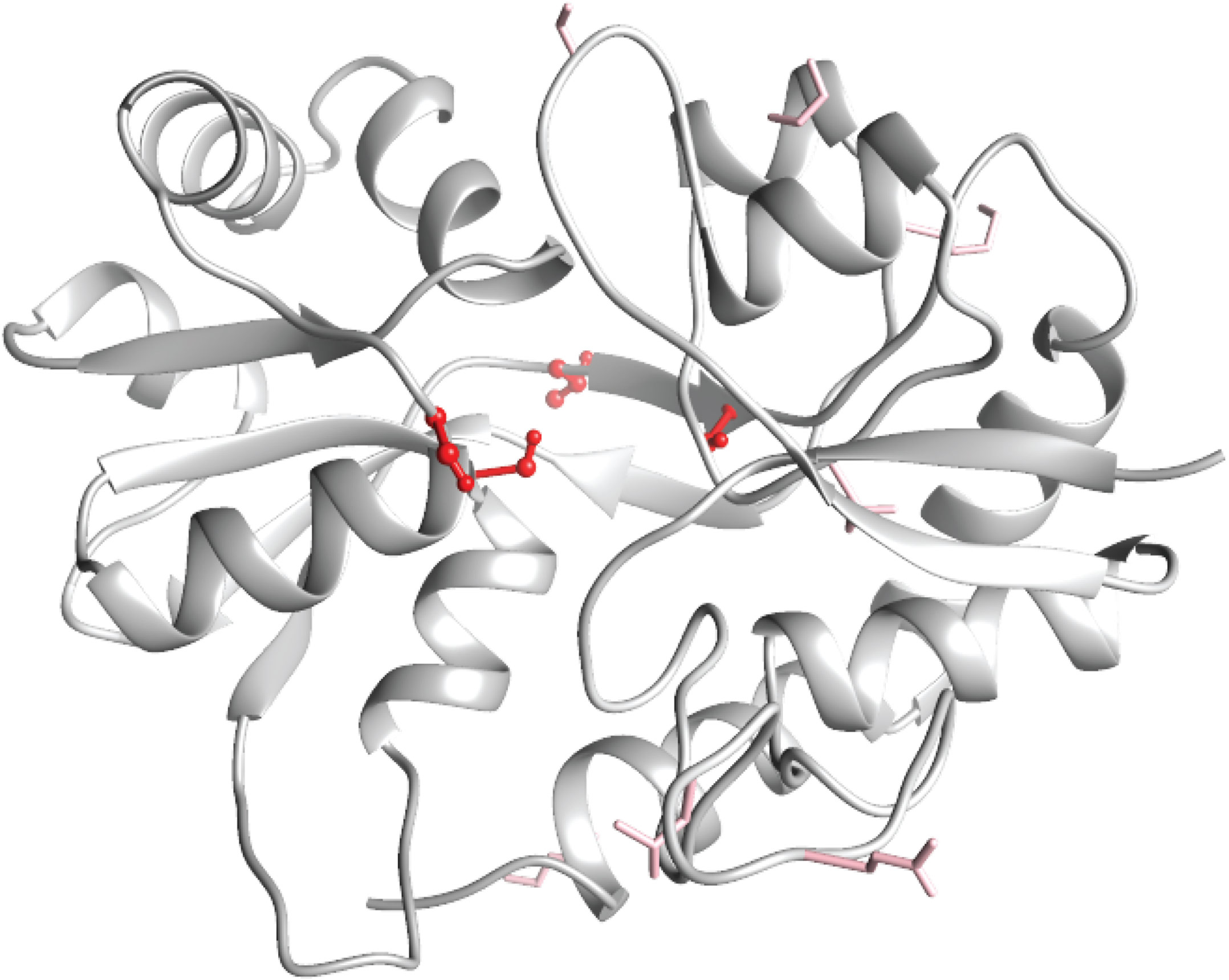
Fig 1. A protein homology model of the odour ligand-binding domain of the Drosophila chemosensory receptor IR75b, highlighting residues important for determining ligand specificity. (Credit: Benoîte Bargeton)
Pheromone signal transduction
Pheromones form one of the main mechanisms by which animals communicate with conspecifics, but how such signals are detected with specificity and sensitivity remains unclear. An important in vivo paradigm for this process is the detection mechanism of the sex pheromone cis-vaccenyl acetate (cVA), which involves a CD36-related transmembrane protein SNMP1, and the receptor OR67d. Through in vivo structure/function analysis of SNMP1, combined with protein homology modelling, and pheromone binding assays, we have provided evidence that SNMP1 forms a tunnel that “funnels” pheromone molecules from the extracellular fluid into the ligand-binding pocket of OR67d in the sensory membranes.
Expanding sensory functions of IRs
Through our own and collaborative work, we have continued to characterise the functions, circuits and behaviours mediated by members of the IR family. Though first identified for their role as olfactory receptors, we have shown that the IR family contributes to a remarkable range of sensory modalities, include taste recognition of amino acids, detection of humidity gradients, cool sensing and temperature-entrainment of the circadian clock. Future work is aimed at determining the molecular mechanism by which members of this protein family can respond to such diverse sensory stimuli.
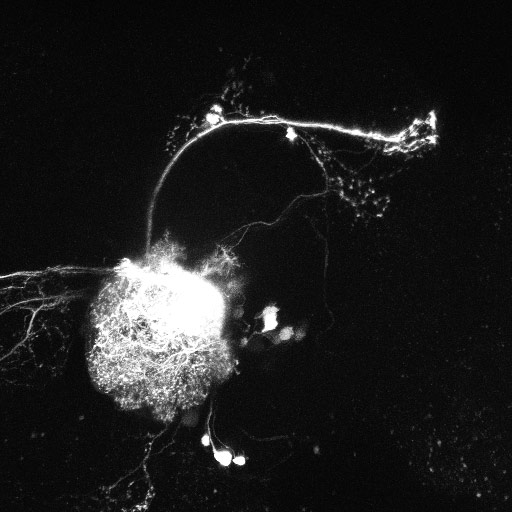
Fig 2. Visualisation of an olfactory interneuron population in the Drosophila brain, labelled by electroporation of a fluorescent dye. (Credit: Lucia Prieto-Godino)
Mechanosensory basis of collective behaviour
During establishment of high-resolution behavioural assays, we discovered a previously unobserved collective behavioural phenomenon in Drosophila, in which group density has a strong influence upon an individual’s response to odours. We showed that this collective behaviour depends upon specific mechanosensory neurons and receptors, which mediate fly-fly encounter-induced locomotion. These findings highlight the importance of social context in the sensory responses of a solitary species, and pave the way to a neural circuit-level understanding of collective behaviour in animal groups.
Chemical communication by trophallaxis in ants
Beyond our Drosophila studies we have investigated a novel type of chemical communication in a social insect, the Florida carpenter ant (Camponotus floridanus). Like other eusocial insects, these ants engage in trophallaxis, a behaviour that permits mouth-to-mouth liquid transfer between members of a colony, which is widely considered to be a simple food-sharing mechanism between adults and from adults to larvae. Through analysis of the protein, RNA and small molecule contents of trophallaxis fluid, we have identified a number of endogenous ant proteins implicated in regulation of juvenile hormone (JH) levels, as well as JH itself. By providing to colonies food supplemented with JH, we found that trophallaxis-mediated “external” hormone supplements can influence brood development. This work raises the possibility that trophallaxis underlies a novel mechanism of inter-individual communication in social insects controlling colony-level phenotypes.

Fig 3. A pair of carpenter ants (Camponotus floridanus) exchanging fluid mouth-to-mouth by trophallaxis. (Credit: Adria LeBoeuf)
Evolution of olfaction
Much of our on-going work aims to define the genetic basis of neural circuit and behavioural evolution. Towards this goal, we compare the olfactory pathways of closely related but ecologically distinct drosophilids, in particular D. simulans and D. sechellia. Like D. melanogaster, D. simulans is distributed worldwide and attracted to diverse fermenting vegetal substrates. By contrast, D. sechellia is endemic to the Seychelles archipelago, and is an ecological “specialist” that is attracted to, and feeds and breeds exclusively on the acid-rich fruit of Morinda citrifolia. We have identified changes in the odour-recognition properties and expression of acid-sensing receptors in D. sechellia and are currently defining the molecular basis of these differences. We are also visualising the neural pathways in which these receptors are expressed, to identify modifications in circuit organisation; subsequently we will use quantitative trait locus mapping to locate the genes responsible for adaptations in circuit development. Finally, we have applied genome-editing technologies to D. sechellia and D. simulans to test the contribution of these olfactory pathways and their species-specific structural and functional properties to the distinct host preferences of these drosophilids.
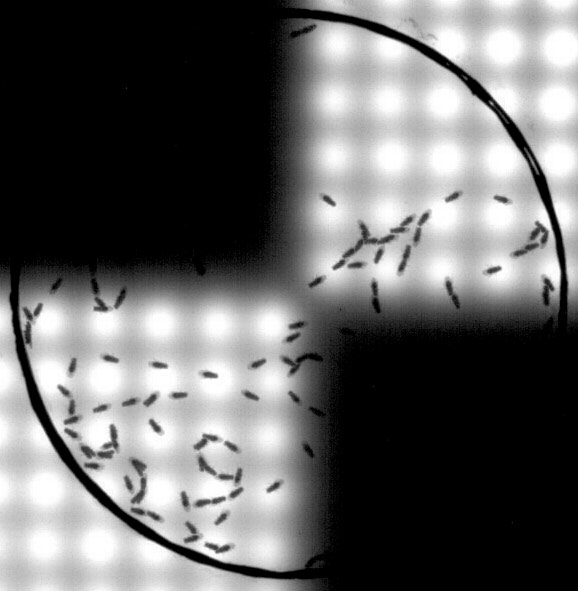
Fig 4. Smelling light: by engineering olfactory neurons to express a red light-sensitive ion channel, the behavioural role of these neurons can be determined by observing whether Drosophila are attracted or repelled by this light source (to which they are blind). (Credit: Lucia Prieto-Godino)

Fig 5. Cover from Trends in Ecology and Evolution (Benton, 2015)
GROUP MEMBERS
Group Leader
Richard Benton
richard.benton@unil.ch
Postdoctoral Fellows
Jacob Roman Arguello
Thomas Auer
Benoîte Bargeton
Adria Le Boeuf
Phing Chian Chai
Lucia Prieto-Godino
Pavan Ramdya
Juan Antonio Sánchez Alcañiz
PhD Students
Jan Armida
Kaan Mika
Marta Scalzotto
Master Student
Raquel Alvarez Ocaña
Technicians
Liliane Abuin
Steeve Cruchet
Giovanna Lucarelli (Zappia)
Visiting Student
Naoko Toshima
Administrative assistants
Annick Crevoisier
Iris Marouani
iris.marouani@unil.ch
