Short Biography
Prof. Lluis Fajas Coll was born in Barcelona, Spain. He studied Biology at the University of Barcelona, where he obtained his Master’s degree. He next moved to the Ernst Boehringer Institute in Vienna, Austria, where he did his PhD studies. After two post-doctoral stages in France, at the laboratory of Prof. Auwerx at Pasteur Institute in Lille and at the IGMM in Dr. Sardet lab in Montpellier, he was recruited as Inserm associated scientist at the IGBMC in Strasbourg. He next was appointed junior group leader with an Inserm Avenir grant in Montpellier. He was recruited in 2012 as Professor and director of the department of physiology at the University of Lausanne. He is now group leader at the CIG, starting in October 2016. The main focus of his research has been the link between cell cycle regulation, proliferation and metabolism in the context of metabolic pathologies, such as obesity and diabetes and in the context of cancer.

LLUIS FAJAS COLL – RESEARCH REPORT
A novel role for cell cycle regulators in the control of metabolism
Metabolic reprogramming is a hallmark of normal physiological homeostasis, but also of pathological conditions. Growing evidences exist about the regulatory crosstalk between metabolic pathways, cell cycle progression and cell division: our laboratory has shown that cell metabolism is reprogrammed through the regulation of metabolic enzymes by various cell cycle regulators. In particular, the Cdk4-Rb-E2F1 pathway controls metabolism of non-proliferative cells, and their activity is altered especially under pathological conditions such as obesity and diabetes.
Taking advantage of tissue-specific conditional knockout mice for E2F1 and for Cdk4, we are now exploring the role of E2F1 and Cdk4 in regulating other major metabolic pathways. In particular, we study the role of E2F1 in gluconeogenesis and cholesterol metabolism in the liver, two processes deregulated in diabetes and in cardiovascular diseases, respectively. In parallel, we are trying to elucidate the function of Cdk4 in thermogenesis in brown adipose tissue, an emerging player in regulating energy expenditure, body weight and global metabolic homeostasis. Some of our major axis of research are as follows:
Cell cycle regulators in the metabolism of normal cells
Our hypothesis is summarized in the following figure.
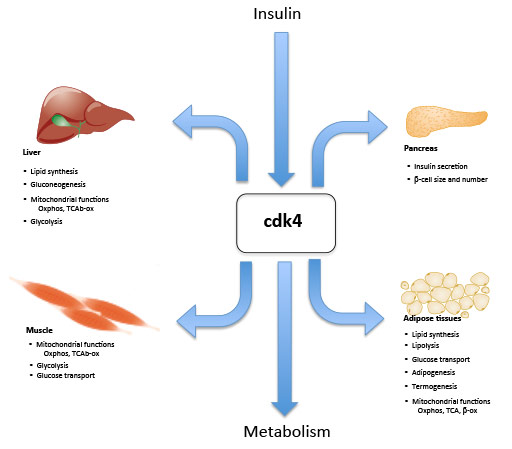
Fig 1. Cdk4 is a major sensor and effector of cellular metabolic homeostasis at the core of insulin signaling, is regulated by insulin and mediates its effects in insulin sensitive tissues.
This hypothesis is supported by our previous observations, showing that Cdk4 is expressed and is active in insulin-responsive tissues, such as white adipose tissue, muscle or pancreatic β-cells under quiescent conditions, suggesting a role of Cdk4 in tissue function beyond the control of proliferation. Cell cycle regulators are key factors in the control of proliferation and cell survival. They have been typically involved in several physiological processes, including development, tissue regeneration or metabolism. Undoubtedly there is cross-talk between the cell cycle and metabolic control. Factors such as nutrition, stress or physical exercise are signaled and translated into proliferative stimuli, but the cell does not usually proliferate in response to these stimuli. Instead, it activates a metabolic response, such as glucose and fatty acid utilization or insulin secretion. Modulation of the activity of the factors that trigger a metabolic pathway in response to proliferative stimuli might therefore open up new perspectives in the control of metabolic diseases such as type II diabetes, obesity and cancer. The most recent analyses performed in my lab using E2F1- and Cdk4-deficient mice show that these proteins are strongly implicated in metabolism in several cell types. Lipolysis, lipogenesis, glucose homeostasis or mitochondrial function are part of the metabolic pathways regulated by E2F1 and Cdk4. We have shown that the Cdk4-Rb-E2F1 network is a sensor of the nutritional and energetic status of the cell. We also showed in another study that the Cdk4-Rb-E2F1 pathway is a negative regulator of energy expenditure, through repression of PGC-1α-mediated transcription. In summary, our results show that these factors are essential regulators of anabolic, biosynthetic processes, blocking at the same time oxidative and catabolic pathways. Our hypothesis is also based on data of my lab that indicate that Cdk4 interacts and phosphorylates proteins involved in metabolic control. Mass spectrometry analyses and two hybrid system experiments have indicated that AMPK and FoxO1 are direct targets of Cdk4.
Cell cycle regulators in the metabolism of cancer cells
Cancer therapy has mostly focused on target signaling pathways of cell proliferation, migration, invasion and survival. However, no standard therapies target metabolism despite the fact that changes in metabolism are a hallmark of cancer cells. The next figure summarizes the global hypothesis of our lab.
The work in our lab concerning this project has been focusing on the following :
1.
Consider lipid synthesis as an essential pathway for cancer cells survival. We show that several signaling pathways, including Wnt and Akt, are repressed in response to lipid synthesis inhibition, whereas a concomitant activation of AMPK is observed.
2.
Lipid synthesis targets are part of a cancer-specific metabolic network controlled by oncogenic factors. We delineate how Cdk4-E2F1 pathways regulate lipid metabolism, and also the relative contribution of such regulation to cancer progression. Our preliminary data indicate that in cancer cells, E2F1 modulates oxidative metabolism, facilitating the glycolytic metabolic switch in these cells. Moreover, E2F1 is essential in regulating the expression of genes implicated in lipid synthesis pathways. Interestingly, oncogenic signaling triggered by E2F1 requires de novo lipid synthesis to induce proliferation of cells.
3.
We found that Cdk4 is a key regulator of cell signaling that influences the activity of major growth mediators, including the mTOR, AMPK or the IGF1R pathways. In particular, we provide evidence that support a direct interaction of Cdk4 with these pathways. We are currently characterizing such interactions.
GROUP MEMBERS
Group Leader
Lluis Fajas Coll
lluis.fajas@unil.ch
Postdoctoral Fellows
Pierre-Damien Denechaud
Albert Giralt Coll
Isabel Lopez-Mejia
Qiuwen Lai
PhD Students
Judit Castillo Armengol
Li Honglei
Laia Martinez Carreres
Anita Nasrallah
Honglei Ji
Technician
Brigitte Delacuisine
Administrative assistants
Nadina Becirovic
Carine Dovat
carine.dovat@unil.ch
